

报 告 人: Prof. Tsunehiro Tanaka(教授、日本京都大学
报告时间: 2022-10-05上午10:00~上午11:30
报告地点: 腾讯会议,会议ID: 159-387-813
组织单位: 王心晨团队
报告题目:Photocatalytic Reduction of CO2 into CO with Water Molecules as an Electron Donor over Photocatalysts
报告时间:2022年10月05日上午10:00-11:30
报告地点:腾讯会议
会议ID: 159-387-813
报 告 人:Prof. Tsunehiro Tanaka(教授、日本京都大学)
报告主持人:Prof. Masakazu Ampo(教授、beat365正版唯一官网&日本大阪公立大学)
报告摘要:
Photocatalytic conversion of CO2 to reduction products, such as CO, HCOOH, HCHO, CH3OH, and CH4, is one of the most attractive propositions for producing green energy by artificial photosynthesis.
Herein, we found that Ga2O3 photocatalysts exhibit high conversion of CO2. Doping of Zn species into Ga2O3 suppresses the H2 evolution derived from overall water splitting and, consequently, Zn-doped, Ag-modified Ga2O3 exhibits higher selectivity toward CO evolution than bare, Ag-modified Ga2O3. We observed stoichiometric amounts of evolved O2 and CO like CO2 splitting. Mass spectrometry clarified that the carbon source of the evolved CO is not the residual carbon species on the photocatalyst surface, but the CO2 introduced in the gas phase. Doping of the photocatalyst with Zn is expected to prevent H2 formation preserving the active site for CO synthesis.
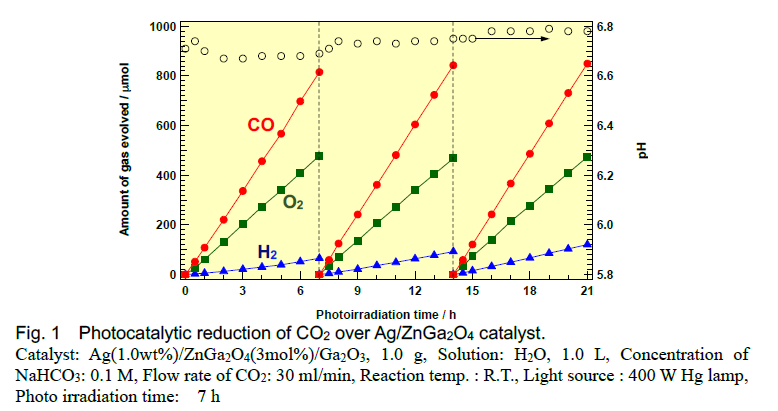
High CO yield could be achieved but simultaneous evolution of oxygen is not preferable for practical use. In order to cut off the evolution of oxygen by oxidation of water molecules, we adopt ammonia NH3 as a sacrificial oxidizing reagent instead of water H2O. In this case, not only cutting off the oxygen evolution but also the formation of N2 and controllable H2 formation could be achieved. Thus, syn-gas formation from CO2, water, and NH3 was realized.
Further, the most active another catalytic system will be introduced.
References:
K. Teramura, Z. Wang, T. Tanaka, et al., Chemistry-A European Journal, 2014, 20, 9906.
Z. Wang, K. Teramura, T. Tanaka, et al., J. Mater. Chem. A, 2015, 3, 11313; ibid, Catal. Sci. Technol.,
2016, 6, 1025.
K. Teramura, K. Hori, T. Tanaka, et al., J. Phys. Chem. C, 2017, 121, 8711.
Z. Huang, K. Teramura, T. Tanaka et al., Chem. Sci., 2017, 8, 5797.
P. Rui, K. Teramura, T. Tanaka, et al. ACS Sustainable Chem. Eng., 2019, 7, 2083.

报告人简介:
Tsunehiro Tanaka教授,现任日本京都大学工程学院分子工程系教授,1987年于日本京都大学化学专业获得博士学位,1987-1990年在美国北海道大学化学系(Department of Chemistry, Faculty of Science, Hokkaido University)做助理教授,1990年进入日本京都大学工程学院工作。1996年在中国兰州物理化学研究所担任客座教授。1996年在意大利都灵大学物理无机材料化学系担任客座学者。曾获得“日本催化协会青年科学家奖”。目前主要从事多相光催化研究;机动车催化剂元素战略研发;催化反应表征研究等。